IP AND COMPLIANCE
Protecting our customers intellectual Property and Confidentiality is a top Priority.We will comply with all aspects of our contracts
- Confidentiality Agreements
- Contracts
- Customer Specifications
- Customer Labeling
- UDI
- Design History File
- Engineering Change Control
- Quality Control
- Customer Segregation
Certifications:
- cGMP(Current Good Manufacturing Practice)
- ISO Standards for Components
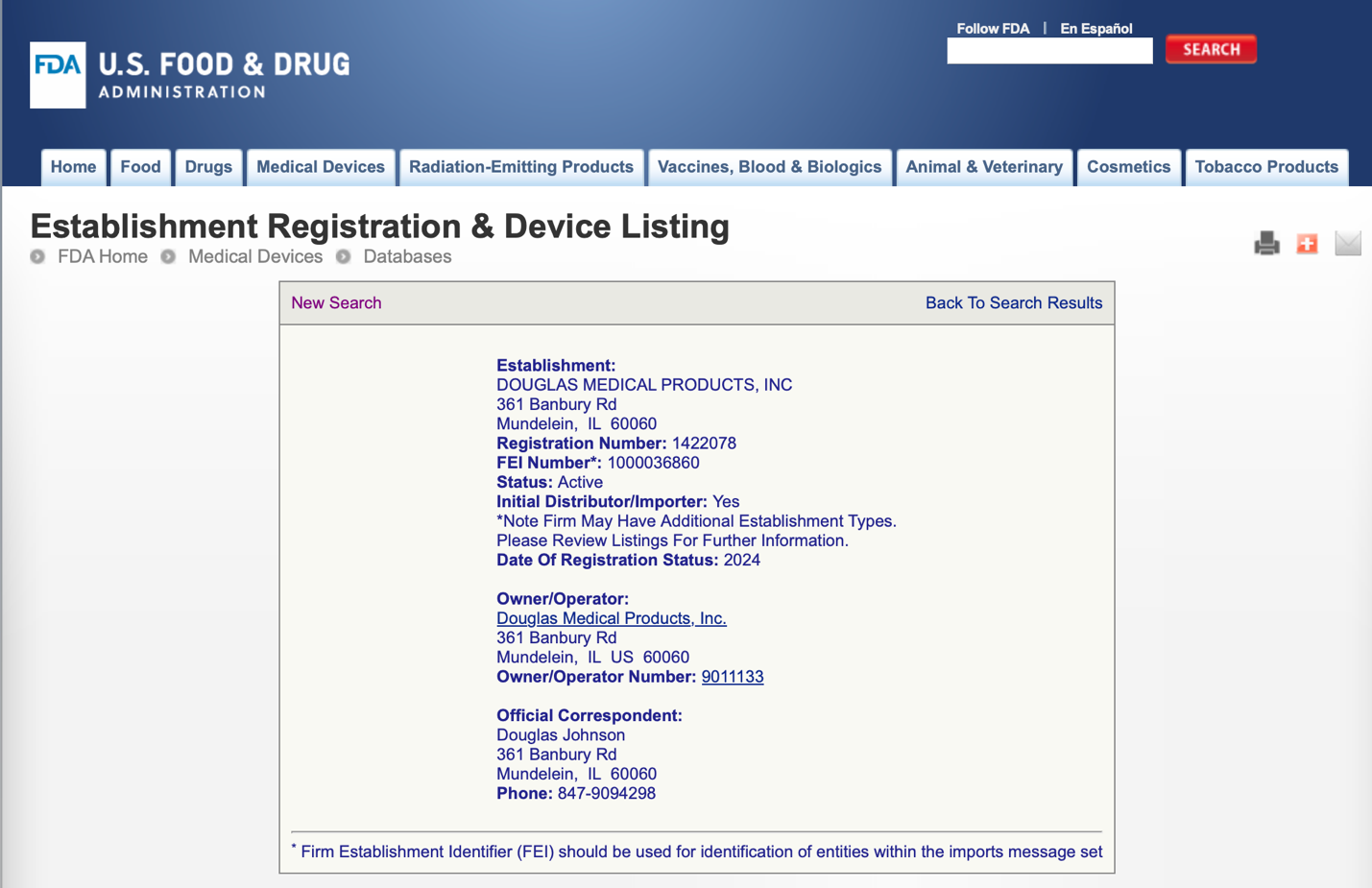
- FDA Registration
- QSR (Quality System Regulation 21 CFR Part 820)
Audits:
We welcome our customers to audit us and our partners facilities
